Background. Myeloid leukemia occurs with a 150-fold increased incidence in children with Down syndrome (ML-DS) and is characterized by distinct disease mechanism and response to treatment. In the context of trisomy 21, somatic GATA1 mutations first initiate preleukemic transient abnormal myelopoiesis, a disorder of fetal liver hematopoiesis. Co-operating events, which target cohesin complex components, epigenetic modifiers or signal transducers including RAS pathway genes (Yoshida 2013, Labuhn M 2019), then drive transformation to ML-DS in 20% of infants with TAM, typically by age 4 years. Abnormal sonic hedgehog signaling was recently associated with trisomy 21 (Galati 2019). Its role in the development of ML-DS is unknown. Hypersensitivity of ML-DS blasts to chemotherapy with cytarabine and other agents accounts at least in part for the more favorable response of ML-DS compared to acute myeloid leukemia of children without DS. Contemporary treatment protocols for ML-DS achieve event-free and overall survival of approx. 89% and 90% at 5 years (Taub 2017). In marked contrast, patients who develop a relapse of ML-DS face a significantly lower probability of survival (EFS 20.9% and OS 22% at 3 years, Raghuram 2023), compared to children with relapsed AML who do not have DS, and appear to be resistant against conventional chemotherapy. Since the few survivors with a relapsed ML-DS are almost exclusively found among patients who first achieve a second remission and then undergo hematopoietic stem cell transplantation, successful identification of agents with efficacy against the blasts of relapsed ML-DS is essential for survival. We tested if blasts of relapsed ML-DS had lost their sensitivity to cytarabine and whether new drugs could be identified that target pathways active in the blasts of ML-DS.
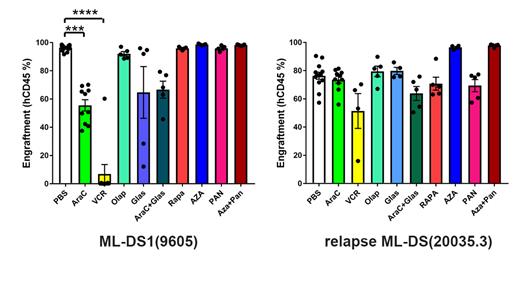
Patients, Materials and Methods. We used patient-derived xenografts (PDX) of human ML-DS and relapsed ML-DS blasts in immunodeficient mice (NSG and NSG-W41) to determine in vivo responses to standard chemotherapeutic agents (cytarabine, vincristine) and novel approaches such as demethylating agents (azacytidine), inhibition of histone deacetylation (panobinostat), mTOR (rapamycin), PARP (olaparib), and sonic hedgehog signaling (glasdegib) alone or in combination (glasdegib + cytarabine; panobinostast + azacytidine). Injection of leukemic cells was performed intrafemorally. Endpoint was inhibition of leukemic engraftment compared to control recipients receiving vehicle only.
Results.Responses of primary and relapsed ML-DS samples to drugs tested in PDX models showed marked heterogeneity. While some ML-DS samples showed resistance to cytarabine at the time of relapse this was not the universal cause of failure of primary treatment of ML-DS and some blasts of relapsed ML-DS retained sensitivity to cytarabine. Some primary and relapsed ML-DS blasts showed unexpected sensitivity to vincristine, a drug more commonly used to treat acute lymphoblastic leukemia. Despite intriguing rationales we observed no responses of primary and relapsed ML-DS blasts to the PARP inhibitor olaparib, mTOR inhibition by rapamycin and sonic hedgehog inhibition by glasdegib.
Conclusions. Loss of hypersensitivity to cytarabine was not universal at the time of ML-DS relapse. Some agents not typically used to treat AML, such as vincristine, showed unexpected efficacy whereas others lacked responses despite a plausible mechanistic rationale. Patient-specific molecular mechanisms underlying relapsed ML-DS are likely to have an impact on drug sensitivity. They should be determined for all patients with relapsed ML-DS to assist identification of targets and selection of drugs in order to establish urgently needed but currently still lacking efficacious treatment for relapsed ML-DS.
Disclosures
No relevant conflicts of interest to declare.